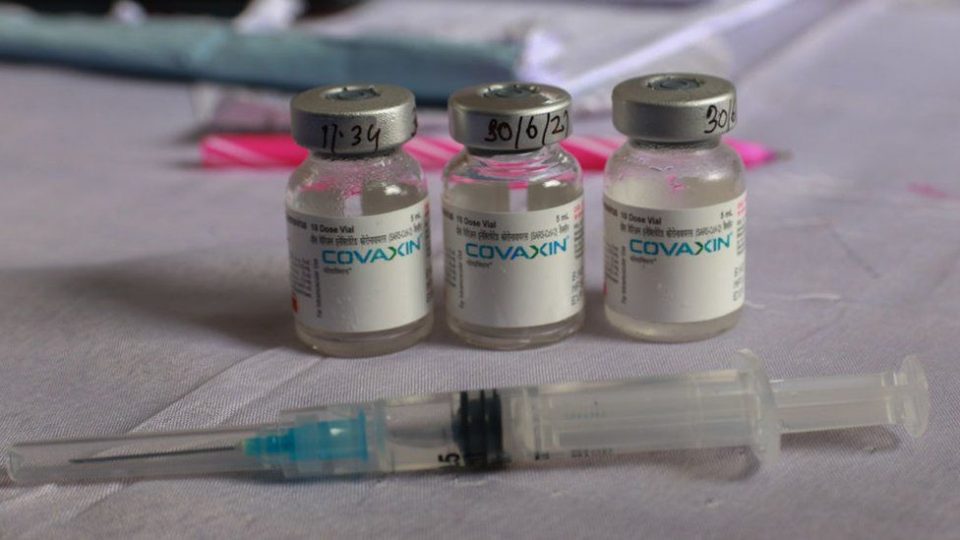
The World Health Organization (WHO) has granted approval for emergency use to India’s government-backed Covid-19 vaccine, Covaxin.
The vaccine was approved in India in January while the third phase of clinical trials was still under way, sparking some concern and criticism.
Bharat Biotech, which makes the vaccine, has since published data suggesting 78% efficacy.
The WHO said in a tweet it believed the benefits far outweighed the risks.
Some experts had pointed to a fast-track approval and incomplete data, but the firm’s chairman, Dr Krishna Ella, said the vaccine was “200% safe”.
The WHO’s expert panel, which authorises emergency approvals, had asked for more data last month while examining the application Bharat Biotech had filed in July.
In its approval it said:
- The vaccine was recommended for use in two doses, with a dose interval of four weeks, in all age groups 18 and above
- Covaxin had 78% efficacy against Covid 19 of any severity, 14 or more days after the second dose, and is extremely suitable for low- and middle-income countries due to easy storage requirements
- Available data on vaccination of pregnant women with the vaccine are insufficient to assess vaccine safety or efficacy in pregnancy
The approval will also be a relief to the tens of millions of Indians who have received the jab – India has administered more than 105 million Covaxin doses so far – and a fillip for Bharat Biotech.
Few countries have recognised Covaxin and India hopes the WHO approval will change that.
How that will play out on travel restrictions for vaccinated Indians remains unclear.
Covishield, the Indian-made version of Astrazeneca, remains the most popular jab, accounting for most of India’s 810 million jabs. It has been approved by the WHO but the UK recognised the jab only after a refusal to do so sparked anger in India.

India has so far fully vaccinated more than 253 million people – about a quarter of its eligible population.
And some 653 million people – about 70% – have had at least one dose of a Covid vaccine so far.