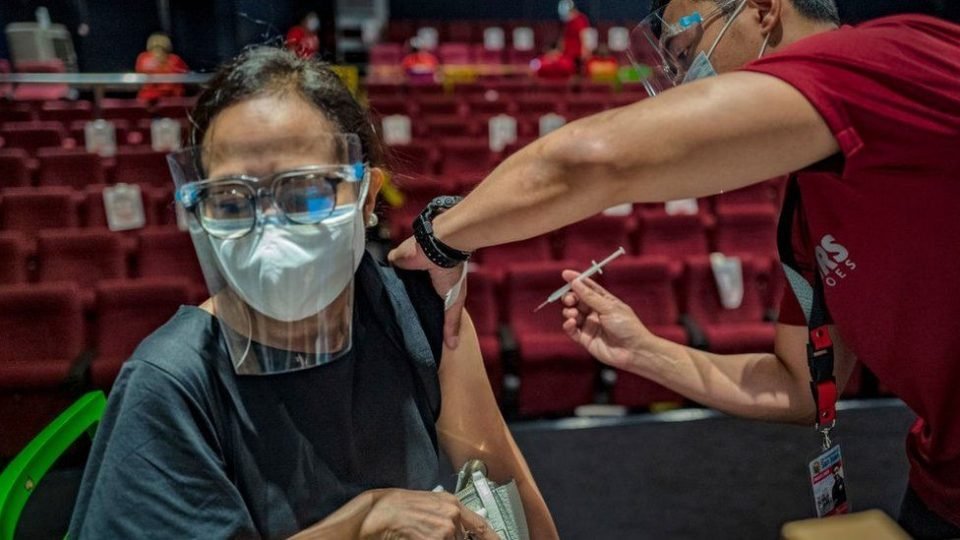
The World Health Organization (WHO) has approved China’s Sinovac Covid vaccine for emergency use.
The WHO said it prevented symptomatic disease in 51% of those vaccinated and prevented severe symptoms and hospitalisation in 100% of samples.
Some evidence and data gaps are still lacking though, according to WHO experts.
It is the second Chinese vaccine to receive the green light from the WHO, after Sinopharm.
The approval opens the door for the jab to be used in the Covax programme, which aims to ensure fair access to vaccines.
The vaccine, which has already been used in several countries, has been recommended for over 18s, with a second dose two to four weeks later.
The emergency approval means the vaccine “meets international standards for safety, efficacy and manufacturing”, the WHO said.
A study in a Brazilian city saw a 95% drop in Covid deaths after it vaccinated almost all of its adults with Sinovac.
Serrana, in the Southeastern Brazilian state of Sao Paulo, is home to 45,000 residents. Once 75% of its population was vaccinated, the number of cases and hospitalisations fell, according to the study.
Brazil has the second deadliest outbreak of Covid infections in the world.
It is hoped that the decision to list the Chinese vaccine for emergency use will give a boost to the Covax initiative, which has been struggling with supply problems.
“The world desperately needs multiple Covid-19 vaccines to address the huge access inequity across the globe,” said Mariangela Simao, the WHO’s assistant director general for access to health products.
“We urge manufacturers to participate in the Covax facility, share their know-how and data and contribute to bringing the pandemic under control,” she said.
China says it has already produced 10 million doses of Covid vaccines for the Covax scheme and that it aims to hit 3 billion doses by the end of the year.
As well as China, the vaccine is already being administered in countries including Chile, Brazil, Indonesia, Mexico, Thailand and Turkey.
One of Sinovac’s main advantages is that it can be stored in a standard refrigerator at 2-8 degrees Celsius.
This means Sinovac is a lot more useful to developing countries which might not be able to store large amounts of vaccine at low temperatures.
The emergency approval came as the heads of the WHO, the World Trade Organisation, the International Monetary Fund and the World Bank appealed for a $50bn (£35bn) investment fund to help end the pandemic.
In a joint statement they said the world had reached a perilous point, and that inequalities in access to vaccines risked prolonging the pandemic, and many more deaths.
They have called for the money to be invested in areas including vaccine production, oxygen supplies, and Covid-19 treatments, ensuring they are distributed fairly.
They also called on wealthy countries to donate vaccine doses immediately to developing nations.