A new coronavirus vaccine has been shown to be 89.3% effective in large-scale UK trials.
The Novavax jab is the first to show in trials that it is effective against the new virus variant found in the UK, the BBC’s medical editor Fergus Walsh said.
The PM welcomed the “good news” and said the UK’s medicines regulator would now assess the vaccine.
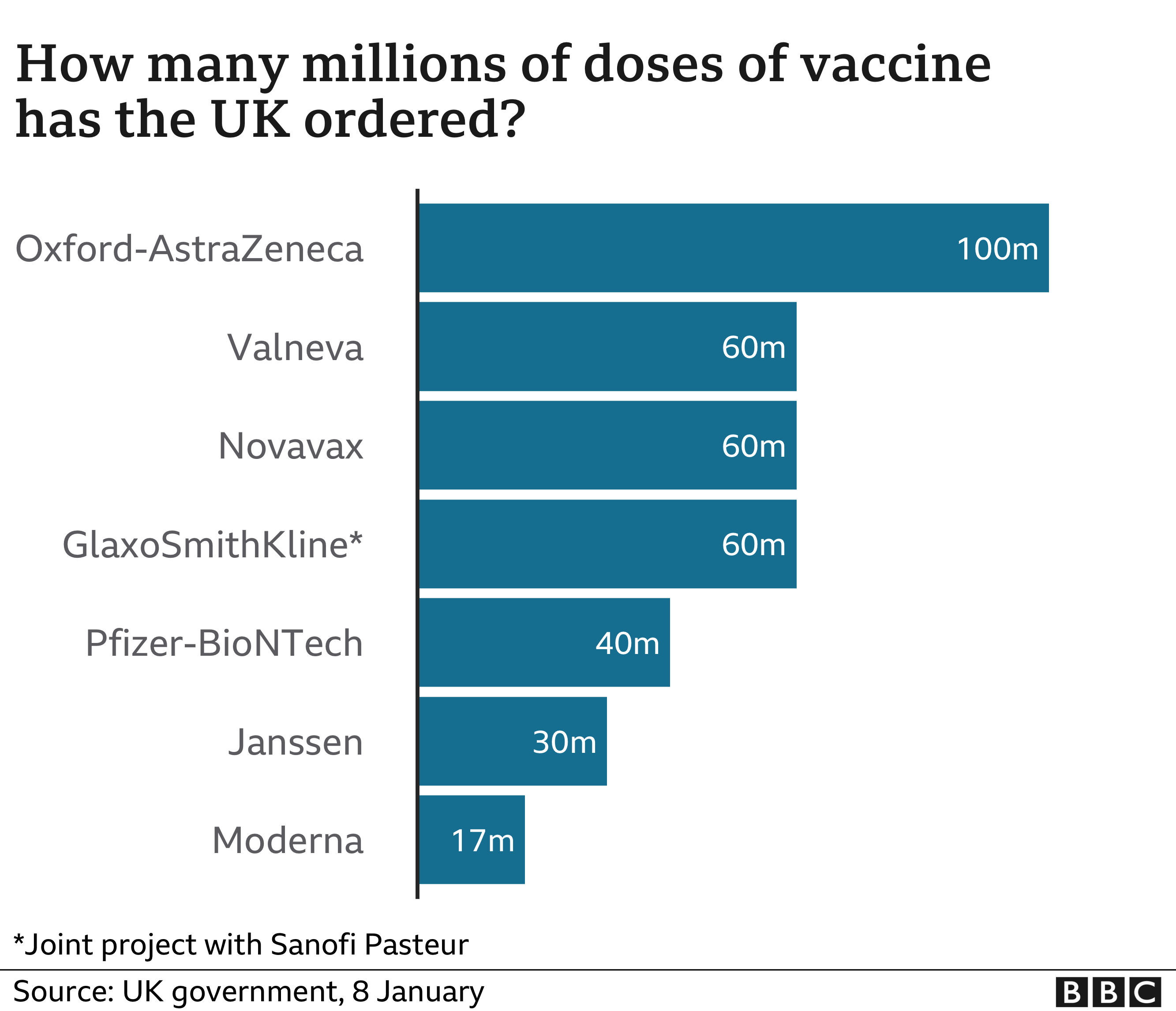
The UK has secured 60 million doses of the jab, which will be made in Stockton-on-Tees in north-east England.
The doses are expected to be delivered in the second half of this year, if approved for use by the Medicines and Healthcare Products Regulatory Agency (MHRA), the government said.
The UK has so far approved three coronavirus vaccines for emergency use – one from Oxford University and AstraZeneca, another by Pfizer and BioNTech, and a third from drug firm Moderna.
The Novavax jab, which is given in two doses, was shown to be 89.3% effective at preventing Covid-19 in participants in its Phase 3 clinical trial in the UK, and around 86% effective at protecting against the new UK variant.
The Phase 3 trials – the final stage before a vaccine is looked at by a regulator – enrolled more than 15,000 people aged between 18-84, of whom 27% were older than 65, US firm Novavax said.
In the South African part of the trial, where most of the cases were the South African variant of the virus, the vaccine was 60% effective among those without HIV.
Stan Erck, chief executive of Novavax, said the results from the UK trial were “spectacular” and “as good as we could have hoped”, while the efficacy in South Africa was “above people’s expectations”.
He told the BBC the manufacturing plant in Stockton-on-Tees should be up and running by March or April, with the company hoping to get approval for the vaccine from the MHRA around the same time.
Minister Lucy Frazer told BBC Breakfast the government could not put an exact timeframe on when the Novavax jab might be approved as the regulation process is “out of our control”.
But the prisons minister added the NHS would be “ready to distribute [the jab] into people’s arms” as soon as supplies become available.
Health Secretary Matt Hancock said the new vaccine would be “another weapon in our arsenal to beat this awful virus”, if approved.
Thanking researches and volunteers who took part in the trials, he added: “I’m proud the UK is at the forefront of another medical breakthrough.”
Prof Paul Heath, chief investigator of the UK Novavax trial, said the findings of the clinical trials were “enormously exciting”, particularly because of the jab’s efficacy against the UK variant.
Peter Openshaw, professor of experimental medicine at Imperial College London, said the findings that the vaccine gave high levels of protection in the UK part of the trial were “excellent” but that the lower level of protection seen in South Africa was “a concern”.


These extremely encouraging trial results suggest another powerful vaccine against coronavirus could soon be within reach.
It works in a slightly different way to the ones that are already available – but does the same job of teaching the body’s immune system to recognise and fight the pandemic virus.
What is more, it appears to be effective against emerging and more infectious variants of coronavirus too – something scientists have feared might not be possible because the vaccines were all designed to match the original virus, not these new, mutated versions.
Protection against illness from the new UK variant was high – around 86%.
Even with the South Africa variant, which has undergone the most worrisome changes, it offered a level of protection similar to that given by flu shots against influenza.
England’s chief medical officer Prof Chris Whitty said, if the jab is approved, it “increases our future resilience” against the virus.
Nadhim Zahawi, the UK government minister responsible for the vaccine rollout, said he was “particularly thrilled” to see the positive results as he had taken part in Novavax’s trials himself.
Labour leader Sir Keir Starmer described the trial results as “fantastic news” and “one more step towards getting Britain vaccinated”.
In total, the UK has ordered 100 million doses of the Oxford-AstraZeneca vaccine and 40 million of the Pfizer-BioNTech vaccine – both of which are currently being rolled out in the UK.
Another 17 million doses of the Moderna vaccine, which was approved by the MHRA in early January, are expected in the spring.
The aim is to give everyone in the top four priority groups – up to 15 million people – a first dose by mid-February.
Pfizer and Moderna vaccines rely on technology that has not been used in previous vaccines, but the Novavax jab uses a more traditional method of recreating part of the spike protein of the virus to stimulate the immune system.
Like the Oxford vaccine, the Novavax jab can be stored at regular fridge temperature – which means it can be distributed more easily.

More than 7.4 million people in the UK have so far received a first dose of a coronavirus vaccine, according to the latest government figures.
Earlier, the prime minister and Public Health England (PHE) defended the use of the Oxford-AstraZeneca jab, after Germany recommended that it should only be given to people aged under 65.
The European Medicines Agency is to decide later whether to approve the vaccine for use across the EU.
Dr Mary Ramsay, PHE’s head of immunisations, said the jab offers “high levels of protection” against Covid-19, particularly against severe illness.
Prof Anthony Harnden, deputy chair of the Joint Committee on Vaccination and Immunisation (JCVI), told BBC Radio 4’s Today programme that Germany has greater supplies of the Pfizer jab than the Oxford one, so its recommendations were made because it wanted to prioritise its larger supply for its elderly population.
Separately, the head of the European Commission has called for the EU’s vaccine contract with AstraZeneca to be published, in a growing row over reduced supplies of their jab.
Kate Bingham, who used to chair the UK’s Vaccine Taskforce, said the reason the UK had a good supply of vaccines compared with other countries was because of its ability to get clinical trials completed quickly and at a high standard through the NHS’s registry – with some 400,000 volunteers signing up “before the US even started their Phase 3 studies”.
She told the Today programme companies in the UK had also been given support to scale up their manufacturing processes quickly.
This means the UK is “far ahead on manufacturing” supplies compared with the US and the EU, despite being a “relatively small player with less buying power”.
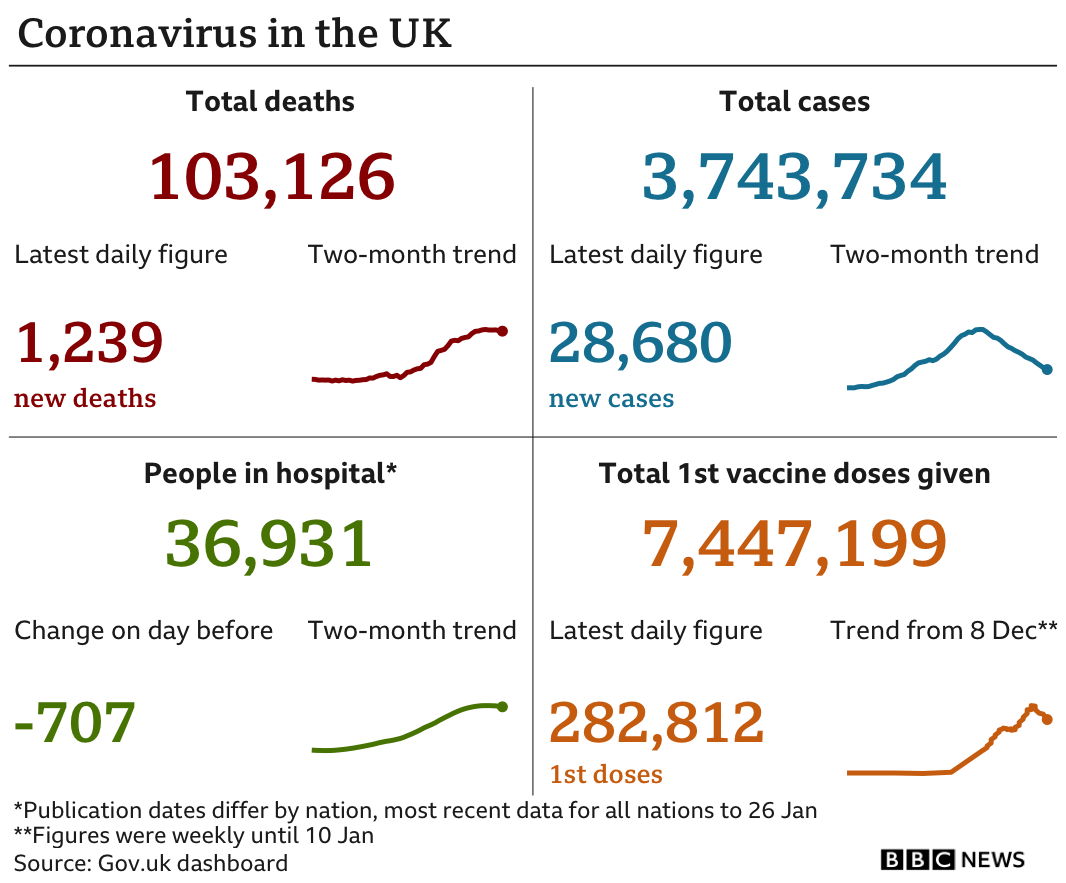
The UK recorded a further 1,239 deaths within 28 days of a positive coronavirus test on Thursday. There have also been another 28,680 new infections.